Methanol: The Game-Changing Solution to Hydrogen Fuel Storage?
? 1) Methanol is vital in the chemical industry and as an energy fuel, usually produced from fossil fuels. Shifting to renewable Methanol, derived from biomass or green hydrogen and CO2, can expand its use as a feedstock and fuel while meeting net carbon-neutral goals.
? 2) Methanol Statistics:
1. Annual production = 98 Mt
2. Current feedstock → fossil fuels
3. Life-cycle emissions = 0.3 gigatonnes (Gt) CO2 p.a.
4. Methanol from fossil fuels releases 1.5 Gt CO2 p.a. by 2050 at 500 Mt p.a.
5. Methanol cost from fossil fuels = USD 100-250 per tonne.
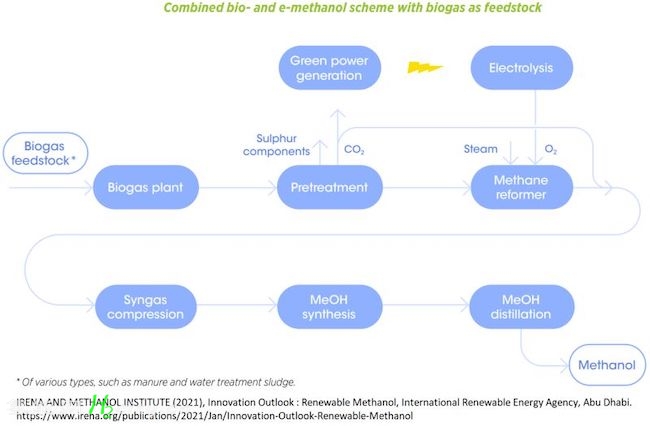
? 3) Renewable Methanol:
1- Bio-methanol from biomass
2- Green e-methanol from captured CO2 and green hydrogen
3- Renewable methanol mostly as bio-methanol =< 0.2 Mt p.a.
4- Anticipated cost of e-methanol by 2050 =< USD 250-630
? 4) Methanol Feedstock:
Natural Gas → 65%
Coal → < 35%
Biomass & renewables → <1%
? 5) Methanol Applications:
Formaldehyde → 25%
MTO → 25%
methyl tert-butyl ether (MTBE) as fuel → 11%
Chloromethanes → 2%
Methylamines → 2%
Acetic acid → 8%
MMA → 2%
Gasoline blending and combustion as fuel → 14%
Biodiesel as fuel → 3%
dimethyl ether (DME) as fuel → 3%
Others → 5%
Total Fuel usage → 31%
? 6) Methanol as fuel:
1] Methanol can fuel conventional ICE, hybrid, and fuel cell vehicles.
2] Liquid Methanol is a cost-effective alternative to hydrogen gas for fuel cell vehicles.
3] Unlike hydrogen, it doesn't require expensive high-pressure storage, making it practical for transport.
4] Methanol is the only liquid fuel used in fuel cell-based transport applications.
5] Maritime application: Currently, more than 20 large ships are powered by Methanol, either in operation or on order.
? 7) Methanol storage and transport:
- Methanol is a liquid at room temperature.
Melting point = -97.8 °C
Boiling point = 64.7 °C
- Methanol is a better liquid energy storage medium than gas and would allow for easy infrastructure transition at low cost.
? 8) Methanol production processes:
1. Renewable methanol
H2O + electricity → H2 + O2 (electrolysis)
CO (renewable) + 2H2 → CH3OH
CO2 (renewable) + 3H2 → CH3OH + H2O
2. Low-carbon methanol
From Methane:
CH4 + H2O ↔ CO + 3 H2
CH4 + 2 H2O ↔ CO2 + 4 H2
CO + H2O ↔ CO2 + H2
Direct oxidation of Methane: 2CH4 + O2 2CH3OH
? 9) My recommendation for transitioning to renewable methanol:
1. Developing and implementing policies that promote the production and use of renewable methanol.
2. Investing in research and development to improve the efficiency and cost-effectiveness of renewable methanol production.
3. Creating public awareness campaigns.
4. Collaborate with policymakers and other stakeholders.
5. Encouraging the use of renewable methanol in transportation and industry.
6. Establishing partnerships with other countries.
✅ My posts reflect my personal perspective, knowledge, experience, and advice.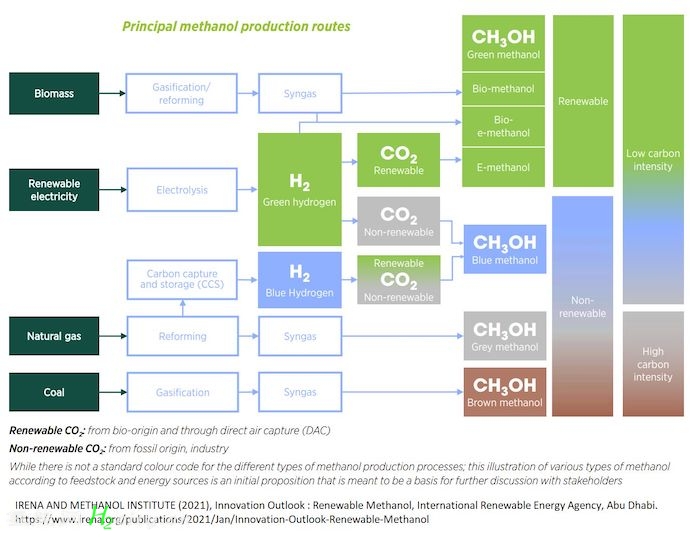
Can methanol solve hydrogen storage and transport challenges?
平台声明:该文观点仅代表作者本人,零碳未来网 系信息发布平台,我们仅提供信息存储空间服务。








发表评论 取消回复